Please contact us for any questions or request for reagents for this structure. |
MGC35130 (SGC Target ID UBC53) - Human Ubiquitin-Conjugating Enzyme of Unknown Function |
|
|
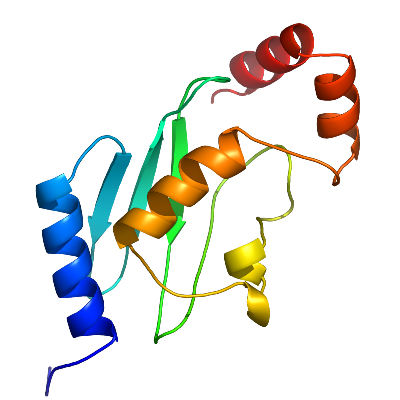
MGC35130 is one of the most divergent members of the E2 family, containing a minimum number of conserved residues. All conserved amino acids except the nucleophilic cysteine are core residues. The domain fold of MGC35130 is clearly that of the ubiquitin conjugase family, but while the amino terminal secondary structural elements align very well with other members, the carboxyl terminal helices (helix-A3 and A4) are positioned significantly away from the conserved position. The large side chain of Tyrosine-140 contributes to the packing of helix-A4, and may be responsible for the tertiary structure divergence. The human genome contains numerous genes encoding ubiquitin-like proteins as well as about forty genes for ubiquitin-conjugating proteins. One of the more intriguing questions concerns specificity, namely, which ubiquitin homolog binds to which conjugating protein(s). As an approach to solving this problem, we aim to determine the structures of all human ubiquitin-conjugating proteins. |
|
|
|
