Please contact us for any questions or request for reagents for this structure. |
ARL8 - Human ADP-Ribosylation Factor-Like 8 |
|
|
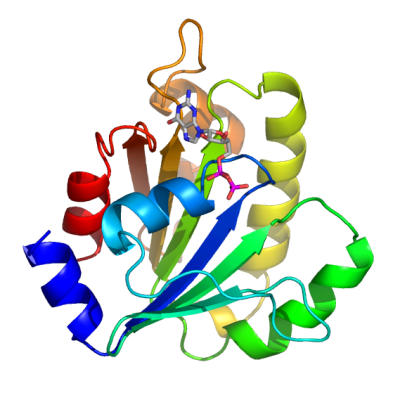
ADP-ribosylation factor (ARF) proteins are RAS-like small G proteins with molecular weights of 20 kDa. They include ARF, ARF-like (ARL) and Secretion-associated and Ras-related (SAR) proteins, all of which are collectively called the ARF family. While ARF and SAR proteins are known to function in membrane trafficking, ARL proteins are not well characterized. The GDP-GTP structural cycle, coupled to recruitment to membranes, has been proposed on the basis of several ARF and ARL structures. When GTP replaces GDP, ARF triggers a conformational change to displace the N-terminal helix, which in turn interacts with membranes. We have solved the structure of ARL8 in complex with GDP. The overall structure of ARL8-GDP shows the inactive conformation as in the other ARF-GDP structures, in which the ordered N-terminal helix is bound to the main body of ARL8. The new structure of ARL8-GDP substantiates the proposal that the membrane recruitment mechanism of the ARF family proteins via the N-terminal helix is linked to the activation of ARF by GTP. |
|
|
|
