Please contact us for any questions or request for reagents for this structure. |
|
AK3L1 - Human Adenylate Kinase 3-Like 1Adenylate kinase (AK), known also as phosphotransferase or myokinase, is a key enzyme participating in cellular metabolism. AK is essential in prokaryotes, which catalyzes the interconversion of three adenine nucleotides in the cell [Tomaselli et al]: MgNTP + AMP Several AK isoforms exist in vertebrates; however their physiological importance is not fully understood. AK1 and AK2 function as ATP:AMP transferases (EC 2.7.4.3), whereas AK3 catalyzes phosphate transfer from GTP to AMP (EC 2.7.4.10). AK1 activity is found in the cytoplasm of skeletal muscles, brain and erythrocytes, AK2 is distributed both in the cytoplasm and the mitochondria while AK3 is associated with mitochondria [Noma et al]. |
|
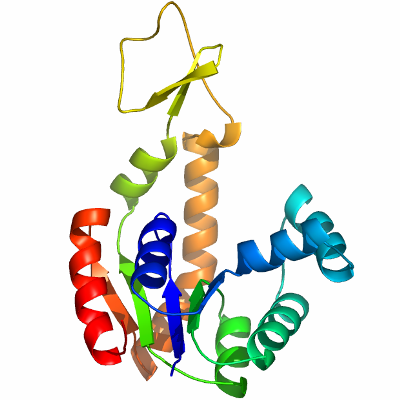
As AK1 and AK2 deficiencies are linked to hemolytic anemia and central nervous system disorders [Unger et al, Cerri et al, Lachant et al], the crystal structure of human AK1 was recently solved by the Structural Genomics Consortium (PDB code 1Z83). Comparison of 17 structures of AKs from different organisms show the structural changes of two domains, which are caused by bound substrates or their analogues [Vonrhein et al]. These different conformational states range between a "closed" and a less well-defined "open" conformation, supporting the hypothesis that the catalytic domain moves considerably during enzyme activity to shield the active site from water in order to avoid ATP-hydrolysis [Jencks et al]. We solved the structure of human AK3-like 1 (AK3L1) in a substrate-free state. The primary sequence of AK3L1 shares ~32% identity to AK1 and AK5, 45% identity to AK2 and 61% identity to AK3. This substrate-free structure, with the future structures of AK3L1 in complex with different ligands, will allow a detailed analysis of structural changes during the enzymatic cycle. | |
References Tomasselli, A.G. and Noda, L.H. (1979) Mitochondrial GTP-AMP phosphotransferase. 2. Kinetic and equilibrium dialysis studies. Eur J Biochem 93 (2), 263-267 Noma, T. et al. (2001) Structure and expression of human mitochondrial adenylate kinase targeted to the mitochondrial matrix. Biochem J 358 (Pt 1), 225-232 Unger, W. et al. (1983) Adenylate kinase activity of cerebrospinal fluid in central nervous system disorders. Eur Neurol 22 (1), 65-69 Cerri, C.G. et al. (1981) Adenylate kinase deficiency and malignant hyperthermia. Hum Genet 57 (3), 325-326 Lachant, N.A. et al. (1991) Hereditary erythrocyte adenylate kinase deficiency: a defect of multiple phosphotransferases. Blood 77 (12), 2774-2784 Vonrhein, C. et al. (1995) Movie of the structural changes during a catalytic cycle of nucleoside monophosphate kinases. Structure 3 (5), 483-490 Jencks, W.P. (1975) Binding energy, specificity, and enzymic catalysis: the circe effect. Adv Enzymol Relat Areas Mol Biol 43, 219-410 |
|
|
|
|

 MgNDP + ADP (N = A or G)
MgNDP + ADP (N = A or G)