Please contact us for any questions or request for reagents for this structure. |
|
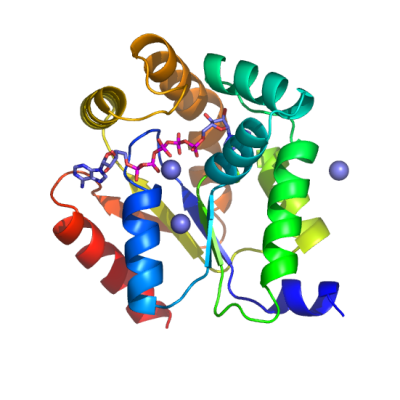
AK1 - Human Adenylate KinaseAdenylate kinase 1 is essential for the maintenance of cellular energetic economy
and is a key enzyme in the synthesis, equilibration and regulation of adenine nucleotides.
AK1 catalyzes adenine nucleotide exchange (ATP + AMP |
|
An intimate relationship of adenylate kinase with mitochondrial respiration,
myofibrillar and membrane ATPases, as well as metabolic sensors
such as ATP-sensitive K+ channels, contributes to efficient regulation of energy metabolism,
membrane excitability, and cell contraction.
The membrane bound form, AK1 beta, is a specific isoform,
which has a presumed role in compartmentalized ATP-GTP homeostasis and a growth-regulatory function.
Genetic deletion of AK1 reduces the cellular energetic economy and increases cell vulnerability to injury. In humans, adenylate kinase deficiency caused by mutations in the AK1 gene has been associated with severe hemolytic anemia and psychomotor impairment. Adenylate kinase deficiency in the erythrocyte is a rare genetic disorder associated with hemolytic anemia. Several mutations have been described ranging from amino acid substitutions, missense mutations and deletions allele, which resulted in nonsperocytic hemolytic anemia. AK1 knockout mice have a decreased potential of myofibers to sustain nucleotide ratios despite upregulation of high energy phosphoryl flux through glycolytic guanolyte and creatine kinase phosphotransfer pathways. They also show decreased tolerance to metabolic stress in heart and skeletal muscle. In addition, decreased AK1 activity has been found in a mouse model for muscular dystrophy (mdx mice) suggesting a direct relationship between the lack of dystrophin and alteration of AK1. | |
References Bianchi, P, Zappa M, Bredi E, Vercellati, and Pelissero G. (199).
A case of complete adenylate kinase deficiency due to a nonsense mutation in AK-1 gene (Arg 107 Carrasco, AJ, Dzeja PP, Alekseev AE, Pucar D, Zingman LV, Abraham MR, Hodgson D, Bienengraeber M, Puceat M, Janssen E, Wieringa B, and Terzic A. (2001). Adenylate kinase phosphotransfer communicates cellular energetic signals to ATP-sensitive potassium channels. Proc Natl Acad Sci 98, 7623-7628. Dzeja, PP, and Terzic A. Phosphotransfer reactions in the regulation of ATP-sensitive K+ channels. (1998). FASEB J 12: 523-529. Schulz GE, Schiltz E, Tomasselli AG, Frank R, Brune M, Wittinghofer A, Schirmer RH. (1986). Structural relationships in the adenylate kinase family. Eur J Biochem. 161, 127-32. |
|
|
|
|

 2ADP) and facilitates transfer of
both β- and γ-phosphoryls in ATP.
The cellular phosphotransfer becomes particularly important in tissues with
an increased energy demand.
Like it has been shown that in heart failure, AK1 expression levels correlate well
with functional recovery in the metabolically compromised heart.
Isoforms of adenylate kinase are found in mitochondria, cytosol, and cellular membranes,
creating an integrated phosphotransfer network.
2ADP) and facilitates transfer of
both β- and γ-phosphoryls in ATP.
The cellular phosphotransfer becomes particularly important in tissues with
an increased energy demand.
Like it has been shown that in heart failure, AK1 expression levels correlate well
with functional recovery in the metabolically compromised heart.
Isoforms of adenylate kinase are found in mitochondria, cytosol, and cellular membranes,
creating an integrated phosphotransfer network.
 Stop, CGA
Stop, CGA