Please contact us for any questions or request for reagents for this structure. |
Py-2-Cys-Prx (PY00414) and Pv-2-Cys-Prx (Pv118545): Plasmodium yoelii and Plasmodium vivax 2-Cys peroxiredoxin |

Peroxiredoxins (Prxs) are antioxidant enzymes catalyzing the reaction: ROOH + 2 e- 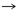 ROH + H2O.
They reduce hydrogen peroxide, peroxynitrite, and organic hydroperoxides to detoxify the cell
and mediate signal transduction.
The three classes of peroxiredoxins include the typical 2-CysPrxs, atypical 2-CysPrxs
and 1-CysPrxs.
The initial categories of Prxs organized them based on the number of cysteines
directly involved in catalysis, thus 2-CysPrx and 1-CysPrx. ROH + H2O.
They reduce hydrogen peroxide, peroxynitrite, and organic hydroperoxides to detoxify the cell
and mediate signal transduction.
The three classes of peroxiredoxins include the typical 2-CysPrxs, atypical 2-CysPrxs
and 1-CysPrxs.
The initial categories of Prxs organized them based on the number of cysteines
directly involved in catalysis, thus 2-CysPrx and 1-CysPrx.
The reaction mechanism comprises two steps: (1) the peroxidatic cysteine (probably as the thiolate, Cys-SP¯) attacks the peroxide substrate and is oxidized to cysteinyl sulfenic acid, Cys-SP-OH, while the RO¯ leaving group is likely protonated; and (2) the Cys-SP-OH is subsequently attacked by the resolving cysteine (CysR) to form a stable disulfide by a condensation reaction. Then, a cellular oxidoreductase reduces the disulfide, completing the catalytic cycle. The SGC has solved the structures of Py-1-cys-Prx (PY04285) and typical Py-2-cys-Prx (PY00414), two peroxiredoxins from the rodent parasite Plasmodium yoelii, as well as the P. vivax orthologue of 2-cys-Prx (Pv118545). These are respectively the orthologues of P. falciparum 1-cys-Prx PF08_0131 and 2-cys-Prx PF14_0368. The structure and description of Py-1-cys-Prx are provided on a different page. In addition, the PDB contains a third member of the plasmodium peroxiredoxin family - antioxidant protein MAL7P1.159 with PDB code 1XIY. Our structure of Py-2-cys-Prx, has the conserved active site motif comprised of the peroxidatic cysteine (Cys50), threonine (Thr47), proline (Pro43) and arginine (Arg125) (shown above with side-chains). Its functional unit is an expected domain-swapped homodimer, a conformation observed in our crystal structure and analytical gel filtration, in which the C-terminus of one monomer, bearing the resolving Cys (circled in blue), forms a disulfide bond with the peroxidatic Cys of the other subunit. This is consistent with our analysis of the protein using mass spectroscopy revealing it to be an oxidized dimer. See
|
|
References Z. A. Wood, E. Schröder, J. R. Harris, and L. B. Poole. Structure, mechanism, and regulation of peroxiredoxins. Trends Biochem. Sci. 28, 2003, 32-40. Kawazu S, Nozaki T, Tsuboi T, Nakano Y, Komaki-Yasuda K, Ikenoue N, Torii M, Kano S. Expression profiles of peroxiredoxin proteins of the rodent malaria parasite Plasmodium yoelii. Int J Parasitol. 2003 Nov;33(13):1455-61. Akerman SE, Muller S.
2-Cys peroxiredoxin PfTrx-Px1 is involved in the antioxidant defence of Plasmodium falciparum.
Mol Biochem Parasitol. 2003 Aug 31;130(2):75-81.
|
|
|
